Educational TechnologyOnline Education
London dispersion forces
Author: Khan Academy via YouTube
Go to Source
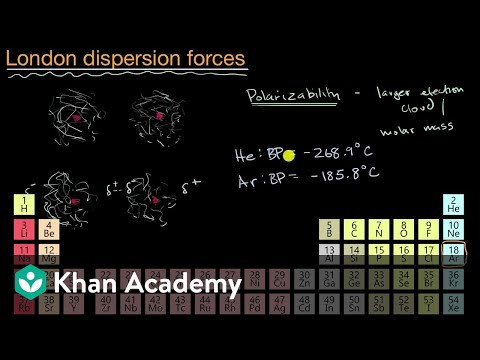
Intermolecular forces due to temporary imbalances in electron distributions that make the atoms/molecules temporarily polar which induce dipoles in neighboring atoms/molecules.
