Atomic spectra | Physics | Khan Academy
Author: Khan Academy via YouTube
Go to Source

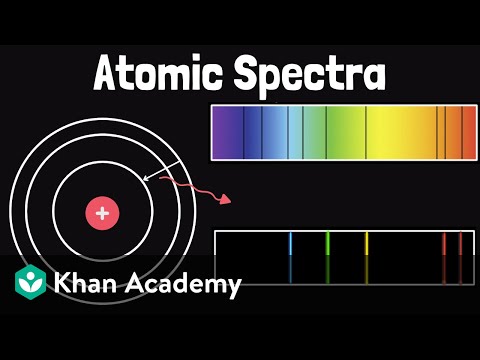
Electrons only exist at specific, discrete energy levels in an atom. If an electron absorbs a photon with energy equal to the difference between two energy levels, the electron will transition to the higher energy level. The atom is then in an excited state. When an electron transitions to a lower energy level, it emits a photon with energy equal to the difference. Every element has a unique emission/absorption spectrum, making atomic spectral lines a valuable tool for identifying elements in distant astronomical objects.
Sections:
00:00 – Intro
00:26 – Electron potential well
03:08 – Orbital shapes
05:37 – Bohr model and energy level diagram
07:09 – Electron excitation and de-excitation
09:36 – Hydrogen’s spectrum
10:54 – Spectral analysis
11:46 – Absorption spectrum
14:11 – Summary
——————
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Volunteer here: https://www.khanacademy.org/contribute?utm_source=youtube&utm_medium=desc
