Beta decay | Nuclear chemistry | High school chemistry | Khan Academy

Author: Khan Academy via YouTube
Go to Source

Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now! https://www.khanacademy.org/science/hs-chemistry/x2613d8165d88df5e:nuclear-chemistry-hs/x2613d8165d88df5e:radioactive-decay/v/beta-decay
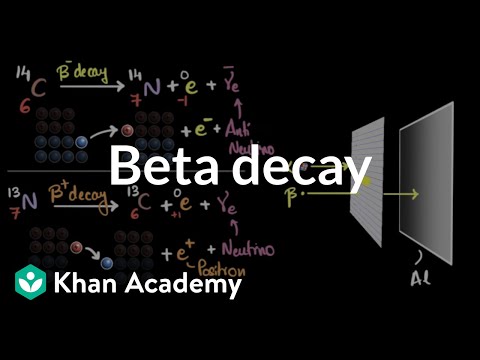
During beta minus decay, a neutron in an unstable nucleus transforms into a proton, releasing a high energy electron and antineutrino in the process. During beta plus decay, a proton in an unstable nucleus transforms into a neutron, releasing a high energy positron and neutrino in the process. Beta particles carry away energy lost by the nucleus and are ionizing radiation.
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate or volunteer today! Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Volunteer here: https://www.khanacademy.org/contribute?utm_source=youtube&utm_medium=desc
