Calculations using Avogadro’s number (part 2) | Chemistry | Khan Academy
Author: Khan Academy via YouTube
Go to Source

Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now!
https://www.khanacademy.org/science/hs-chemistry/x2613d8165d88df5e:stoichiometry-and-the-mole/x2613d8165d88df5e:mole-calculations/v/calculations-using-avogadro-s-number-part-2
Practice converting from grams to moles to molecules, formula units, and atoms through these four example problems. Grams are the standard unit of mass in the laboratory, while moles are used in calculations to relate mass to the number of particles. This system of units allows for communication between the microscopic world of atoms and the macroscopic world we interact with daily.
Sections:
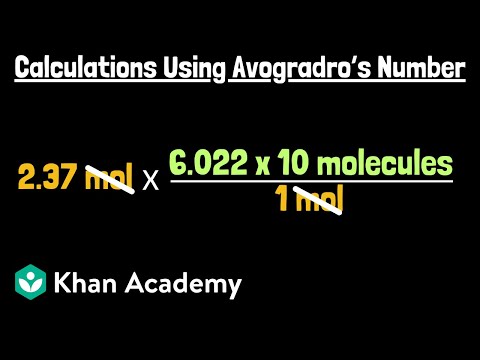
00:00 – Convert moles to molecules of glucose
03:07 – Convert moles to number of hydrogen atoms in glucose
06:29 – What is a formula unit?
08:40 – Convert mass to moles to formula units in CaCO3
12:49 – Calculate the number of oxygen atoms from moles of CaCO3
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate or volunteer today! Donate here: https://donate.khanacademy.org/give/419869/#!/donation/checkout
Volunteer here: https://www.khanacademy.org/contribute?utm_source=youtube&utm_medium=desc
